-
 chevron_right
chevron_right
Tiny “nano-sponges” inspire killer moves in 2023 Dance Your PhD winning video
news.movim.eu / ArsTechnica • 20 March, 2023 • 1 minute
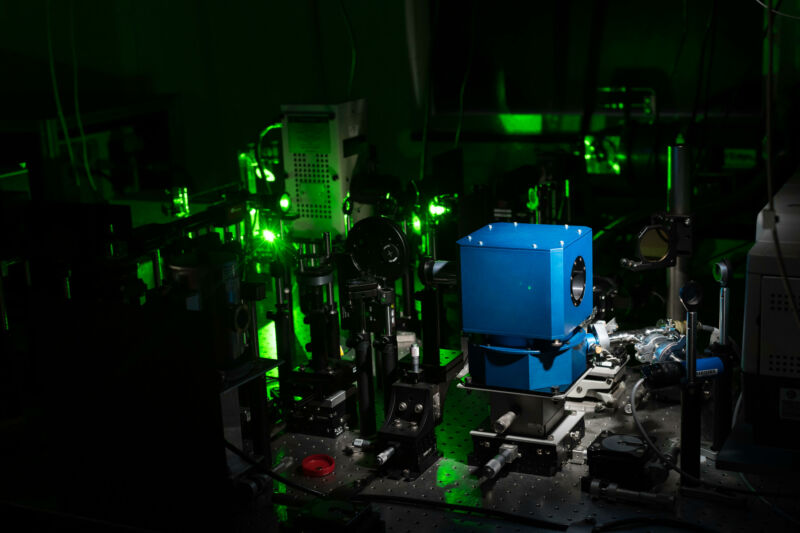
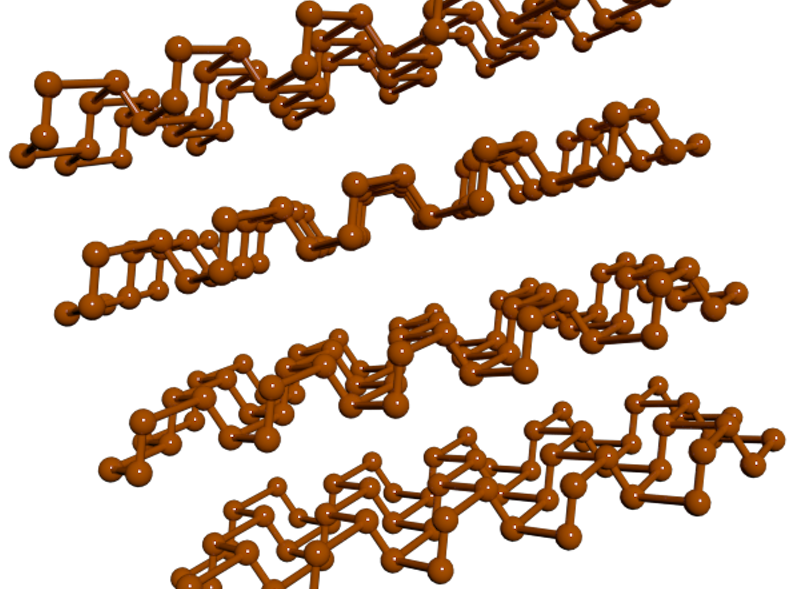
Checkers Marshall’s award-winning dance depicted electrons moving around in crystalline materials that have a variety of applications.
University of Oregon chemist Checkers Marshall took top honors in the 2023 Dance Your PhD contest, combining hand fans, blue balloons, and original lyrics to make a dance video explaining their work on "nano-sponge" materials for use in carbon capture and drug delivery. Other winning videos provided creative takes on how local trees in the Amazon rainforest produce a protective hormone in response to drought; diffusing ions at the nanoscale, illustrated with a tango; and an artificial intelligence model called PsychGenerator that aims to bring personality and mental health attributes to AI.
As we've reported previously , the Dance Your PhD contest was established in 2008 by science journalist John Bohannon. It was previously sponsored by Science magazine and the American Association for the Advancement of Science (AAAS) and is now sponsored by AI company Primer, where Bohannon is the director of science. Bohannon told Slate in 2011 that he came up with the idea while trying to figure out how to get a group of stressed-out PhD students in the middle of defending their theses to let off a little steam. So he put together a dance party at Austria's Institute of Molecular Biotechnology , including a contest for whichever candidate could best explain their thesis topics with interpretive dance.
The contest was such a hit that Bohannon started getting emails asking when the next would be—and Dance Your PhD has continued ever since. It's now in its 15th year. There are four broad categories: physics, chemistry, biology, and social science, with a fairly liberal interpretation of what topics fall under each. Winners were chosen from 28 entries submitted from 12 different countries. All category winners receive $500, while Marshall, as the overall champion, will receive an additional $2,000. And the contest has a new sponsor this year: Sandbox AQ , an Alphabet spinoff focused on tackling large problems by bringing together artificial intelligence and quantum technologies.